Screening and Diagnosis of ID in HF
Challenges With the Diagnosis of Iron Deficiency in Heart Failure
Iron deficiency (ID) is a very common comorbidity in patients with heart failure (HF; both acute and chronic), but overall awareness is low, and the diagnosis of ID is challenging in this patient population. The diagnostic criteria for ID in patients with HF remains an area of active research and disagreement, as shown by the different criteria used to define ID across HF trials.1,2 ID has traditionally been associated with anemia in HF patients, despite the fact that iron status does not strongly correlate with red cell indices in this population. Awareness of ID in HF also remains low, even among specialists, as highlighted by a survey during our own symposium on this topic at the 2020 American Heart Association (AHA) Scientific Sessions. During this symposium, 52% of participants indicated that they rarely assess iron status in HF patients, 14% responded that they have never assessed iron status, and only 10 to 24% do so frequently or all the time.3 As such, these results seem to support prior reports of delayed diagnosis and missed opportunities to address ID in HF.1
IRON DEFICIENCY VERSUS IRON DEFICIENCY ANEMIA
Iron is an essential component of body homeostasis and a key component for erythropoiesis in the bone marrow, and as such, ID is naturally linked with anemia. This condition, often referred to as iron deficiency anemia (IDA), can accompany chronic diseases with an increased inflammatory burden (e.g., chronic kidney disease, HF, inflammatory bowel disease, rheumatoid diseases). However, ID and IDA do not always coexist, and the pathophysiological impact of ID independent of anemia is consequential.4
In patients with HF, ID is much more prevalent than IDA. Approximately 25 to 45% of HF patients have ID without anemia.5 Up to 68% of chronic HF patients have ID,6 and in men and women with acute HF, rates are also high at 57% and 79%, respectively.7 In HF with preserved ejection fraction (HFpEF), ID prevalence rates may be slightly higher than HF with mid-range (HFmrEF) or reduced EF (HFrEF).8 Regardless of anemia status, ID negatively impacts exercise capacity, and is associated with increased acute care utilization, death, and reduced quality of life (QoL) in patients with HF.9 In HFmrEF and HFrEF, ID is associated with higher rates of HF hospitalization, heart transplantation, and increased mortality.9 In HFpEF, ID is also associated with decreased exercise capacity as well as lower QoL.9 Regardless of hemoglobin levels, correction with IV iron reduces hospitalizations, improves QoL, exercise capacity, and patient symptoms.10 A growing body of evidence also suggests that ID may not be a mere comorbidity of HF, but rather an important contributor to HF pathophysiology and disease progression.9 This new data further underscores the importance of ID screening, diagnosis, and treatment in patients with HF, whether or not these patients present with anemia.
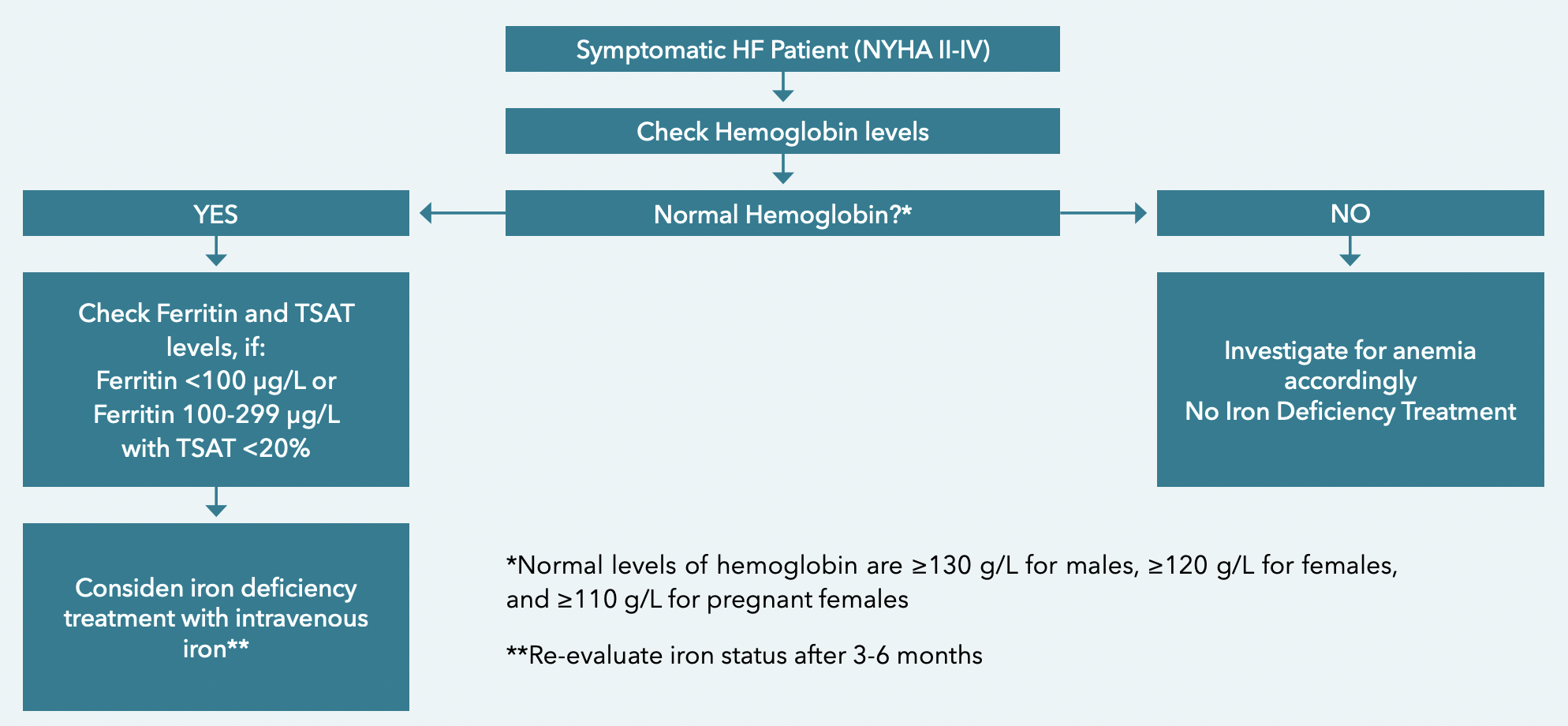
DIAGNOSING IRON DEFICIENCY IN HEART FAI LURE
PERSPECTIVES AND FUTURE DIRECTIONS
References
- von Haehling S, Ebner N, Evertz R, Ponikowski P, Anker SD. Iron Deficiency in Heart Failure: An Overview. JACC Heart Fail. 2019;7(1):36-46. doi:10.1016/j.jchf.2018.07.015
- Ponikowski P, Jankowska EA. Targeting Iron Deficiency in Heart Failure: Existing Evidence and Future Expectations. Circ Heart Fail. 2021;14(5):e008299. doi:10.1161/CIRCHEARTFAILURE.121.008299
- CMHC data on file – Satellite Symposium at 2020 American Heart Association. Addressing the Impacts of Iron Deficiency in HF: Advances in Diagnosis and Management. November 15, 2020.
- Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J. 2013;34(11):816-829. doi:10.1093/eurheartj/ehs224
- Beavers CJ, Ambrosy AP, Butler J, et al. Iron Deficiency in Heart Failure: A Scientific Statement from the Heart Failure Society of America. J Card Fail. 2023;29(7):1059-1077. doi:10.1016/j.cardfail.2023.03.025
- Masini G, Graham FJ, Pellicori P, et al. Criteria for Iron Deficiency in Patients With Heart Failure. J Am Coll Cardiol. 2022;79(4):341-351.doi:10.1016/j.jacc.2021.11.039
- Cohen-Solal A, Damy T, Terbah M, et al. High prevalence of iron deficiency in patients with acute decompensated heart failure. Eur J Heart Fail. 2014;16(9):984-991. doi:10.1002/ejhf.139
- Becher PM, Schrage B, Benson L, et al. Phenotyping heart failure patients for iron deficiency and use of intravenous iron therapy: data from the Swedish Heart Failure Registry. Eur J Heart Fail. 2021;23(11):1844-1854. doi:10.1002/ejhf.2338
- Alnuwaysir RIS, Hoes MF, van Veldhuisen DJ, van der Meer P, Grote Beverborg N. Iron Deficiency in Heart Failure: Mechanisms and Pathophysiology. J Clin Med. 2021;11(1):125. doi:10.3390/jcm11010125
- Savarese G, von Haehling S, Butler J, Cleland JGF, Ponikowski P, Anker SD. Iron deficiency and cardiovascular disease [published correction appears in Eur Heart J. 2023 May 7;44(18):1607]. Eur Heart J. 2023;44(1):14-27. doi:10.1093/eurheartj/ehac569
- Anand IS, Gupta P. Anemia and Iron Deficiency in Heart Failure: Current Concepts and Emerging Therapies. Circulation. 2018;138(1):80-98. doi:10.1161/CIRCULATIONAHA.118.030099
- Al-Naseem A, Sallam A, Choudhury S, Thachil J. Iron deficiency without anaemia: a diagnosis that matters. Clin Med (Lond). 2021;21(2):107-113. doi:10.7861/clinmed.2020-0582
- WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. Geneva: World Health Organization; 2020.
- Grote Beverborg N, Klip IT, Meijers WC, et al. Definition of Iron Deficiency Based on the Gold Standard of Bone Marrow Iron Staining in Heart Failure Patients. Circ Heart Fail. 2018;11(2):e004519. doi:10.1161/CIRCHEARTFAILURE.117.004519