IMPACT OF ID IN HEART FAILURE
OF IRON DEFICIENCY
Reduced exercise capacity
Poor prognosis independently of anemia and left ventricular ejection fraction
Imparied health-related quality of life (HRQoL)
Iron deficiency and exercise intolerance in heart failure
Iron deficiency in patients with stable systolic heart failure has been observed to be associated with submaximal exercise capacity as well as endurance.
In hematopoietic tissues, including erythrocytes, immune cells, and platelets

In nonhematopoietic tissues, including myocardium, adipose tissue, liver, kidneys, and brain





In hematopoietic tissues, including erythrocytes, immune cells, and platelets
In nonhematopoietic tissues, including myocardium, adipose tissue, liver, kidneys, and brain
Anemia
Reduced O² (myoglobin) Reduced tissue oxidate capacity Reduced energetic effiency Anaerobic metabolism Mitochondrial dysfunction
Reduced O² carrying capacity (hemoglobin)
Symptoms related to reduced O² transportation (i.e. reduced maximal performance)
Symptoms related to reduced tissue iron deficiency (i.e. reduced endurance)
In hematopoietic tissues, including erythrocytes, immune cells, and platelets





In nonhematopoietic tissues, including myocardium, adipose tissue, liver, kidneys, and brain
In hematopoietic tissues, including erythrocytes, immune cells, and platelets
In nonhematopoietic tissues, including myocardium, adipose tissue, liver, kidneys, and brain





Iron deficiency and exercise intolerance in heart failure
Iron deficiency in patients with stable systolic heart failure has been observed to be associated with submaximal exercise capacity as well as endurance.
In hematopoietic tissues, including erythrocytes, immune cells, and platelets





In nonhematopoietic tissues, including myocardium, adipose tissue, liver, kidneys, and brain





In hematopoietic tissues, including erythrocytes, immune cells, and platelets
In nonhematopoietic tissues, including myocardium, adipose tissue, liver, kidneys, and brain
Anemia
Reduced O² carrying capacity (hemoglobin)
Symptoms related to reduced O² transportation (i.e. reduced maximal performance)
Reduced O² (myoglobin) Reduced tissue oxidate capacity Reduced energetic effiency Anaerobic metabolism Mitochondrial dysfunction
Symptoms related to reduced tissue iron deficiency (i.e. reduced endurance)
In hematopoietic tissues, including erythrocytes, immune cells, and platelets





In nonhematopoietic tissues, including myocardium, adipose tissue, liver, kidneys, and brain
In hematopoietic tissues, including erythrocytes, immune cells, and platelets
In nonhematopoietic tissues, including myocardium, adipose tissue, liver, kidneys, and brain





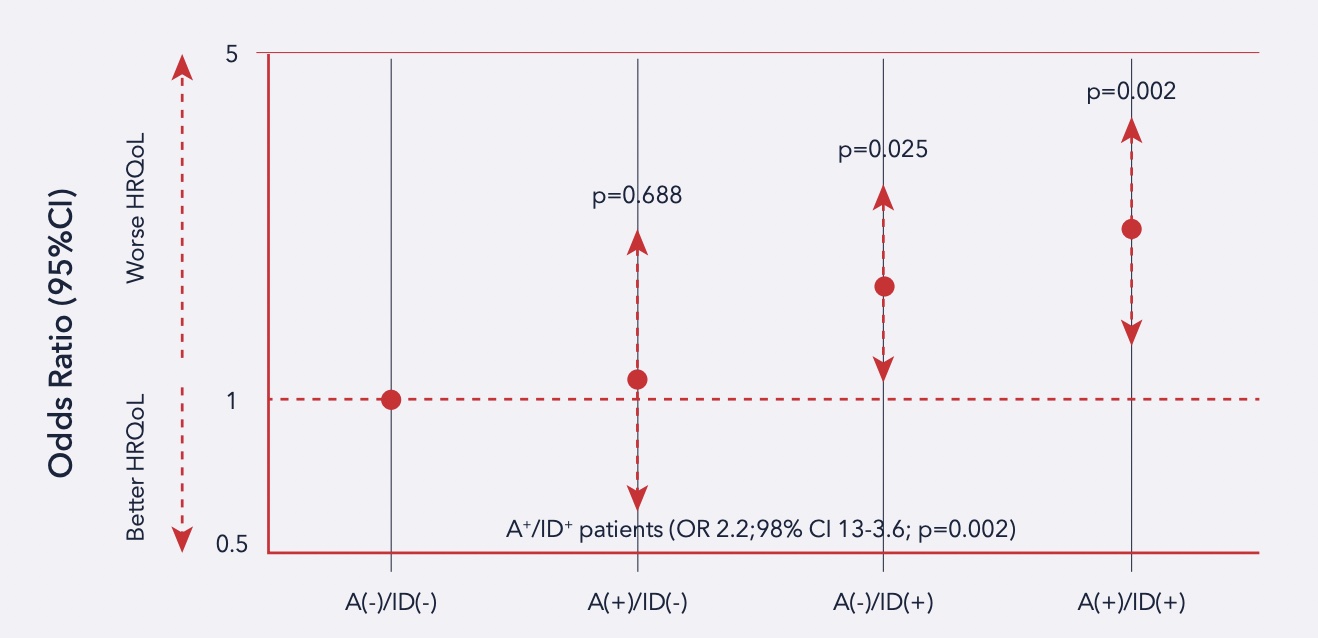
IRON DEFICIENCY is a key determinant of health-related quality of life in heart failure, regardless of anemia status
Patients impacted by chronic heart failure (CHF) present significant impairment of health-related quality of life (HRQoL). The role of iron in energy metabolism could be the link between ID and HRQoL.
Iron deficiency and
prognosis in heart failure
Studies have found that in patients with systolic HF, ID was a strong independent predictor of death as well as heart transplantation.
Iron deficiency and
prognosis in heart failure
Studies have found that in patients with systolic HF,
ID was a strong independent predictor of death as well as heart transplantation.
Kaplan-Meier curves reflecting 3-year event-free survival rates
in 546 patients with systolic heart failure with vs. without iron deficiency.
Download this article as a PDF
REFERENCES:
Van Veldhuisen, Dirk J., et al. “Anemia and iron deficiency in heart failure: mechanisms and therapeutic approaches.” Nature Reviews Cardiology 8.9 (2011): 485-493.
Lewis, Gregory D., et al. “Oral iron therapy for heart failure with reduced ejection fraction: design and rationale for oral iron repletion effects on oxygen uptake in heart failure.” Circulation: Heart Failure 9.5 (2016): e000345.
Jankowska, Ewa A., et al. “Iron deficiency: an ominous sign in patients with systolic chronic heart failure.” European heart journal 31.15 (2010): 1872-1880.
Comín‐Colet, Josep, et al. “Iron deficiency is a key determinant of health‐related quality of life in patients with chronic heart failure regardless of anaemia status.” European journal of heart failure 15.10 (2013): 1164-1172.
Anand, Inder S., and Pankaj Gupta. “Anemia and iron deficiency in heart failure: current concepts and emerging therapies.” Circulation 138.1 (2018): 80-98.